Medical and Healthcare Criteria
Materials for medical and the healthcare industry must meet more stringent criteria than perhaps other industries as there are regulations that mesh with required performance criteria for various healthcare applications. This document provides insight to regulations, known material performance criteria, and related medical/healthcare considerations that should be considered when applying materials to an injection molding process for part production.
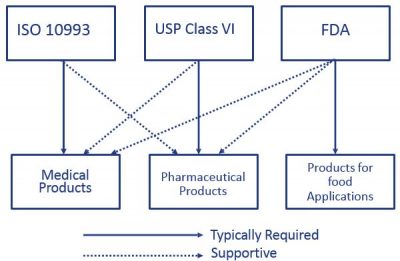
When considering products impacted by healthcare or medical regulations such as medical products, pharmaceutical products and products for food applications, ISO 10993, USP Class VI, and FDA regulations come to mind. Out of three categories medical products require ISO 10993 compliance and are also supported by USP Class VI and FDA regulations. Criteria for healthcare product performance are key drivers behind regulatory focus:
- Biocompatibility – Testing standards are defined by ISO 10993:1, USP Class VI for Plastics
- Product life cycle – Long-term properties (up to 3+ years) reusables and as molded properties for single use
- Sterilization method – Steam Autoclave, High Energy Gamma Radiation, Ethylene Oxide Gas
- Cleaning/Disinfecting – Chemical resistance varies greatly among plastics and is largely dependent on molecular structure
- Retention of properties – Strength and toughness for the life of the product
Biological evaluation and biocompatibility testing of medical devices and materials. The requirements imposed on the biological qualification of medical products in accordance with ISO 10993 depend not only on the type of medical product, but at the same time also on the place of use (skin, mucous membrane, blood, tissue), on the intended function (contact with body surfaces, internal body contact, implantable product), and on the period of use (< 24 hours, < 30 days, unlimited).
The United States Pharmacopeia Convention (USP) is an organization for the evaluation of packaging used for pharmaceutical products. It complies with standards relating to quality, purity and identity, and checks products manufactured world-wide and generally sold, consumed or used in the USA. The USP is a fundamental requirement specifically for pharmaceutical products and their manufacturing technologies. However, for medical products the role of the USP is only to provide a supportive statement for qualification.
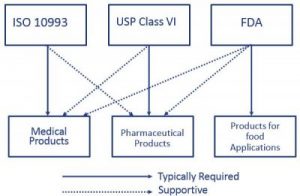
Alongside the evaluation of a material’s suitability for contact with food, FDA compliance is frequently also used in the field of medical technology to provide users with important information on risk assessment. However, it is not a binding requirement for the use of materials in the medical or pharmaceutical field. The American Food and Drug Administration (FDA) assesses the suitability of materials for direct and indirect contact with food. Raw materials, additives and properties of plastics are specified by the FDA in the “Code of Federal Regulations” CFR 21. Materials that meet the relevant requirements are regarded as FDA compliant.
Sterilization methods fall into three groups – gamma, steam, and EtO.
- Gamma irradiation is an ionizing radiation sterilization technique that involves exposing materials for sterilization to gamma rays. Cobalt-60 is the most common gamma radiation source used for industrial ionizing radiation sterilization. Sterilizing radiation dosage is measured in either Grays (Gy) or rads (Radiation Absorbed Dose).
- Steam Sterilization, also known as autoclaving, involves generating or injecting saturated steam into a pressure chamber at a temperature range of 121-148 °C (250-300 °F) at 15 PSI for a period of time sufficient to provide sterilization. Some plastics are degraded by autoclaving.
- Ethylene Oxide gas (EtO) is frequently used to sterilize materials that are otherwise too sensitive to heat or radiation sterilization. Many plastics fall into this category and EtO sterilization is frequently used for single use medical devices made of plastic. EtO gas requires careful handling because of its flammability and how toxic it is. Strict handling requirements and a technically complex sterilization process makes EtO suitable primarily for large volume sterilizations.
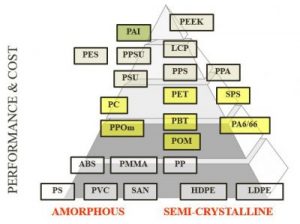
As to chemical resistance, generally semi-crystalline polymers have better chemical resistance than amorphous polymers. Resistance is affected by concentration, temperature, exposure time, exposure type (single sided, immersion, etc.), and stress levels in the part itself. Cost considerations also may impact your selection of material for your medical project. Consider the pyramid below to identify your material candidates.
Material Specifics
Now consider the various materials applicable to medical products and the injection molding process. Each material contains pros and cons, is compliant with or challenged by regulations, and may pass or be constricted by sterilization method.
Polystyrene (PS) | |
| Pros | Cons |
|
+ High stiffness + Very good surface gloss + Excellent transparency + Low melt viscosity / easy process ability |
– Tends to be brittle – Poor chemical resistance – Poor UV resistance
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals – ISO 10933 Approvals + FDA Approvals |
Styrene Acrylonitrile (SAN) | |
| Pros | Cons |
|
+ High strength and stiffness + Excellent dimensional stability + Good chemical resistance + Good resistance to cyclic temperatures + High transparency and gloss |
– Tends to be brittle
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Polypropylene (PP) | |
| Pros | Cons |
|
+ Low density + Excellent fatigue life (living hinge) + Excellent chemical resistance + Low coefficient of friction + Excellent chemical resistance + Good Clarity |
– Poor UV resistance – Difficult to bond
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
+ USP Class VI Approvals – ISO 10933 Approvals + FDA Approvals |
Acrylic (PMMA) | |
| Pros | Cons |
|
+ Excellent optical clarity + Excellent UV resistance + High stiffness + High gloss |
– Poor chemical resistance – Susceptible to stress cracking
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Polycarbonate (PC) | |
| Pros | Cons |
|
+ Very high impact / toughness + Transparency / clarity + Good temperature resistance + Dimensional stability / low warpage + Good mechanical properties + Good creep resistance + Good electrical properties + Can be flame retardant, UV resistant |
– Poor chemical / hydrolysis resistance – Notch sensitivity
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals – FDA Approvals |
CoPolyester | |
| Pros | Cons |
|
+ Outstanding impact resistance + Exceptional clarity + Good chemical resistance + Dimensional stability / low warpage + High gloss + BPA free + Not manufactured with plasticizers + Good gamma resistance |
– Fair UV
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Methyl methacrylate/acrylonitrile/butadiene styrene (MABS) | |
| Pros | Cons |
|
+ Transparency + Resistance to chemicals and stress cracks + Excellent surface quality + Higher heat resistance + Ease of processing + Extensive medical agency approvals |
– Poor UV
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Styrene methyl methacrylate copolymers (SMMA) | |
| Pros | Cons |
|
+ Extreme clarity and transparency + Excellent flow properties + Compliant with FDA/EU food regulations + Resistant to alcohol + Hydrophobic (no pre-drying) |
– Fair chemical resistance – Low impact
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Methyl methacrylate butadiene styrene (MBS) | |
| Pros | Cons |
|
+ Excellent clarity and toughness + Superior flow properties + Easy processing + Meets USP class VI + Good resistance to detergents/solutions |
– Fair chemical resistance
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Styrene butadiene copolymers (SBC) | |
| Pros | Cons |
|
+ Crystal-clear transparency + Excellent toughness + Designed to blend with GPPS + Easy, efficient and versatile processing + Cost-effective solutions |
– Fair chemical resistance – Poor UV
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Polysulfone (PSU)/Polyethersulfone (PES) | |
| Pros | Cons |
|
+ High strength + High HDT 325-400°F + Long term CUT 320-360°F + Low creep + Good chemical resistance + Excellent hydrolytic stability + Transparent |
– Poor solvent resistance – Poor UV resistance
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
– USP Class VI Approvals – ISO 10933 Approvals + FDA Approvals |
Polyphenylene Sulfide (PPS) | |
| Pros | Cons |
|
+ High strength and stiffness + High HDT (260°C / 500°F) + Long term CUT 200°C / 392°F + Excellent chemical resistance + Good electrical properties + Excellent dimensional stability + Inherently self-extinguishing (UL 94 V0) |
– Low toughness
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Liquid Crystal Polymer (LCP) | |
| Pros | Cons |
|
+ High strength and stiffness + High HDT (270°C / 518°F) + Long term CUT 220°C / 428°F + Excellent chemical resistance + Very high melt flow + Inherently self-extinguishing (UL 94 V0) |
– Low weld-line strength – Expensive
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Thermoplastic Polyurethane (TPU) | |
| Pros | |
|
Polyether-based TPU + Excellent hydrolytic stability + Low-temperature flexibility + Microbial resistance + Resistance to weak acids/bases
|
Polyester-based TPU+ Oil/solvent resistance + Weather (UV) resistance + Abrasion resistance + High-temperature resistance + Enhanced mechanical properties |
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide – Steam Autoclave |
– USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Liquid Crystal Polymer (LCP) | |
| Pros | Cons |
|
+ High strength and stiffness + High HDT (270°C / 518°F) + Long term CUT 220°C / 428°F + Excellent chemical resistance + Very high melt flow + Inherently self-extinguishing (UL 94 V0) |
– Low weld-line strength – Expensive
|
| Sterilizations | Regulation |
|
+ Gamma Radiation (1000 Mrad) + Ethylene Oxide + Steam Autoclave |
+ USP Class VI Approvals + ISO 10933 Approvals + FDA Approvals |
Finally, consider the need for Antimicrobial with a product such as SANAFOR which offers a safe and affordable way to effectively control the growth of micro-organisms, adding value to your end products. Benefits of applying antimicrobials include a broad spectrum antimicrobial technology (bacteria, fungi, molds, mildew, yeasts, algae), cost‐effectiveness, durable protection, safe and easy to use during polymer processing, full compatibility with a broad range of polyolefin polymers, good thermostability, and broad regulatory approvals.
Interested in obtaining more advice? Reach a Technical Engineer at sales@xcentricmold.com, or call (586) 589-4636